By: Chioma Madonna Ndukwu
Shock Decision as US-Linked Vaccine Trial in Africa Is Abruptly Cancelled
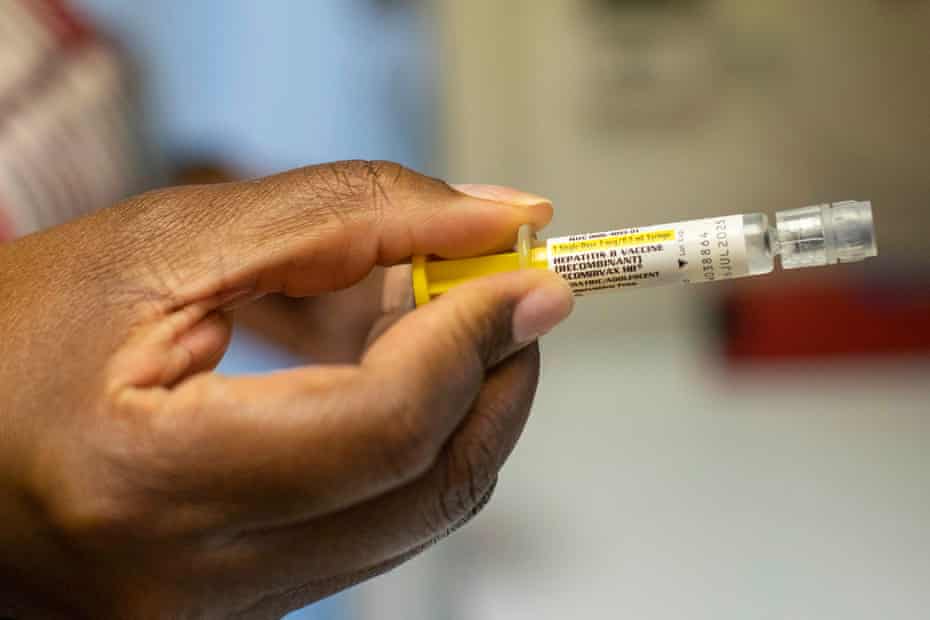
A controversial medical study involving newborn babies in Guinea-Bissau has been suddenly halted, triggering global attention and intense debate across the international health community.
The trial, which focused on hepatitis B vaccination for infants, was scheduled to begin earlier this month but was stopped before full implementation.
Officials at Africa’s Centres for Disease Control and Prevention (Africa CDC) confirmed the cancellation, describing the project as deeply problematic in its original form.
Senior Africa CDC official Yap Boum announced the decision at a press briefing, saying the study could not proceed in its current design.
“The study has been cancelled,” Boum told journalists. “Africa needs strong scientific evidence to guide health policy, but that evidence must be produced within accepted ethical standards.”
A country facing a silent epidemic
Hepatitis B remains one of the most serious public health threats in Guinea-Bissau.
Nearly 18% of adults and about 11% of infants under one year old are infected with the virus. Children who contract hepatitis B at birth are far more likely to develop chronic liver disease, cirrhosis and liver cancer later in life.
Although the hepatitis B vaccine is widely recognised as safe and effective, access remains limited in Guinea-Bissau.
The country currently vaccinates babies at six weeks of age due to supply challenges, but plans to introduce vaccination at birth nationwide by 2027.
The cancelled study was designed to observe health outcomes among newborns who received the vaccine at birth compared with those who did not.
Medical experts, ethicists and human rights advocates strongly criticised the trial after details of its design emerged.
The study planned to randomly provide the vaccine to only half of participating newborns, leaving the other half unprotected — despite the vaccine being proven to prevent a potentially deadly disease.
Critics warned that the approach would knowingly expose thousands of infants to avoidable infection.
“This is not how modern medicine is supposed to work,” said Dr. Paul Offit, an infectious disease specialist at the Children’s Hospital of Philadelphia. “You don’t deny children a life-saving vaccine when you already know it works.”
He compared the project to past medical scandals in which vulnerable populations were used for experimentation.
Health experts across the continent say the cancellation marks a major moment in Africa’s push for ethical, locally driven medical research.
Dr. Boghuma Titanji, an assistant professor of medicine at Emory University, said the decision shows that African institutions are becoming more confident in rejecting exploitative research practices.
“This is a win for advocacy and for the protection of African children,” she said. “Africa should not be treated as a testing ground simply because vaccines are scarce.”
She added that while more clinical trials are needed in Africa, they should be led by African scientists and shaped by African health priorities.
Officials in Guinea-Bissau have indicated that discussions around a redesigned trial may continue.
However, Africa CDC insists that any future study must comply fully with international ethical standards and prioritise the safety of newborns.



 UNICEF, Anambra Govt. guarantee safety in Measles-Rubella vaccination exercise
UNICEF, Anambra Govt. guarantee safety in Measles-Rubella vaccination exercise  Strengthening Nigeria-EU Cooperation: CCCD, IDDRI, FMITI Set to Host Ukama Platform Workshop in Nigeria
Strengthening Nigeria-EU Cooperation: CCCD, IDDRI, FMITI Set to Host Ukama Platform Workshop in Nigeria  New Year, New Habits: 10 Simple Health Changes That Actually Stick
New Year, New Habits: 10 Simple Health Changes That Actually Stick  Maduro’s Removal Sparks Power Shift as Interim Leader Addresses US
Maduro’s Removal Sparks Power Shift as Interim Leader Addresses US  Footage Triggers Uproar After Doctor Is Seen Attacking Patient in Hospital Ward
Footage Triggers Uproar After Doctor Is Seen Attacking Patient in Hospital Ward  TODAY IN HISTORY – 23rd Dec, 2025 – Africa World News
TODAY IN HISTORY – 23rd Dec, 2025 – Africa World News  The Same God Who Settled 2023 Will Settle Abia 2027 — Otti Replies Kalu
The Same God Who Settled 2023 Will Settle Abia 2027 — Otti Replies Kalu  AFCON 2025: Full List of Award Winners as Senegal Lift Trophy in Morocco
AFCON 2025: Full List of Award Winners as Senegal Lift Trophy in Morocco  Ibeh Ugochukwu Bonaventure on Troco Technology: Building Trust Where Nigerians Once Took Risks
Ibeh Ugochukwu Bonaventure on Troco Technology: Building Trust Where Nigerians Once Took Risks